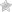Plants known as ‘hyperaccumulators’ can tolerate and transport metals, storing them just under the epidermis of their leaves. Just a few grams, but enough at field scale to consider “cultivating ores”, says Guillaume Echevarria, a specialist in hyperaccumulators from the University of Lorraine, France. The researcher is co-founder of start-up business Econick, which specialises in the phytoextraction of nickel.
Until now, these plants have mainly been of interest for their ability to decontaminate soils polluted with heavy metals like zinc, cadmium, copper, cobalt, lead, thallium etc. But some of the metals absorbed can be used. In 2020, Econick signed a contract with the steelmaking company Aperam to biosource nickel, which is necessary for the production of stainless steel.
A symbolic gesture? Not necessarily. “‘Agromining’ is a very niche industry but all metal economists will tell you that the demand for nickel will be such in the coming years that we cannot turn our back on any economically sustainable source,” notes Mr Echevarria. Demand for the metal is on the rise largely due to the expected surge in electric mobility vehicles, as battery production is particularly nickel-intensive. A further challenge is “the enormous CO2 cost of metals”.

A Pycnandra acuminata tree from New Caledonia with a bluish sap colored by nickel ions.
One tonne of nickel on 4 ha
Although it can’t compete with industrial extraction, the amount of metal that can be extracted from a field is impressive. The shrub phyllanthus rufuschaneyi, cultivated by Econick, absorbs 250kg/ha of nickel per year. “In theory, it will be possible to meet Aperam’s needs, provided we sow several thousand hectares,” says Mr Echevarria. The metal is not a renewable resource, but is abundant in the soil and revegetation of industrial residues (mining tailings) will help complete the circle.
“The plants we currently use are used in their natural state but breeding efforts have begun,” says Mr Echevarria. Breeders are focussing on the plant’s behaviour – in particular its speed of growth, which is more important than the metal yield. For ‘phytomining’ to be profitable, perennials need to be able to regrow quickly after each harvest: “These species, like phyllanthus rufuschaneyi, have a delicate biology and the sowing costs are high. We can’t resow them every year.

Growth of the cadmium and zinc hyperaccumulator Thlaspi caerulescens on metal-polluted industrial soil and unpolluted agricultural soil. (INRAE – SCHWARTZ Christophe)
Zinc derived from plants
In the north of France, Mr Echevarria is also working on decontaminating market garden soil, leading to the production of zinc. This is because some plants, like arabidopsis halleri, hyperaccumulate several metals. One of the projects is located near a former industrial site where vegetables systematically exceed cadmium standards. “We want to make sure that we don’t cut the vegetable production line. We are trying to produce healthy vegetables planted between rows of hyperaccumulators that would protect the crops from excess metals. We’re in the experimental phase.”
As with nickel, extraction relies on burning plants before treating them with chemicals: The ashes are “washed” several times and the deposits collected. The zinc yield is more modest, but the techno-economic analysis demonstrates that, alongside the ecological benefit it offers, heat cogeneration makes production profitable.
The process also has nutritional significance for humans. “Some species can accumulate up to 2% of their biomass in zinc – in soils where there are normal concentrations of this metal,” says Mr Echevarria. Zinc is one of the world’s leading human mineral deficiencies. “It could be an interesting source, rather than consuming zinc produced from chemical substances.”
Hyperaccumulating plants: A fascinating biology
Mainly cruciferous vegetables can tolerate toxicity and have specific storage systems for hyperaccumulating metals. In nature, these traits provide them with a wealth of advantages: Resistance to being eaten (low palatability) and drought stress (water efficiency), as well as natural biochemical defences (creation of toxic litter that keeps competitors away).
Another advantage could be more efficient photosynthesis thanks to UV filtering by metals, although this hypothesis is yet to be validated. The nickel ion wavelength would, for example, provide a barrier against wavelengths that reduce the effectiveness of chlorophyll. This more efficient photosynthesis would therefore compensate for the high metabolic cost of storing the metal in the leaves.